Dexcom Risk, Integrity, & Compliance / Compliance Program
Compliance Program
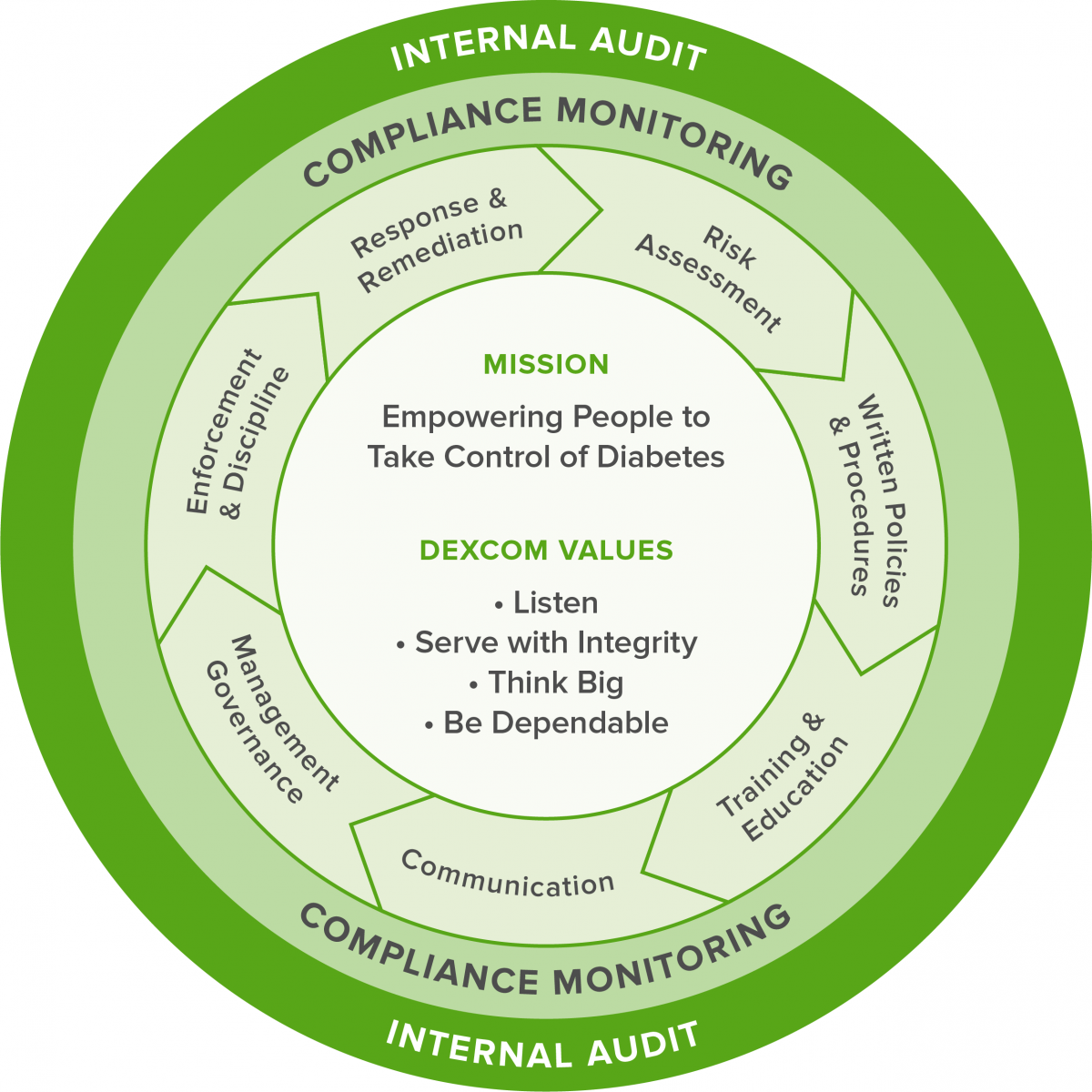
Dexcom is committed to conducting its business affairs ethically and in compliance with all applicable laws and regulations. To that end, we have established a formal Corporate Compliance Program.
The Compliance Program applies to all Dexcom workforce members, including full-time and temporary employees, officers, directors, interns, contractors, and anyone else who conducts business on Dexcom’s behalf. The Compliance Program is intended to promote a culture of quality and transparency and the prevention of improper financial incentives, actual or apparent conflicts of interest, and business practices that unnecessarily increase the costs of healthcare or compromise patient safety.
Dexcom’s Compliance Program Components:
Dexcom’s Compliance Program is led by the Chief Compliance Officer, who is an integral part of the Company’s Risk and Integrity leadership structure. The Chief Compliance Officer reports to the Chief Risk Officer and provides regular updates to Dexcom’s Board of Directors, or an appropriate subcommittee thereof, and Compliance Committee, which provides strategic direction and oversight of compliance risk and implementation of the Program.
Policies, Procedures, and Standards of Conduct
Dexcom’s Compliance Program includes written policies, procedures, and standards, including our Code of Conduct and Business Ethics. These standards help us comply with relevant laws and regulations, industry codes, and ethical requirements.
Critical to the success of the Compliance Program is each workforce member’s understanding of the polices, procedures, and standards that have been established to support the Program. Both at hire and annually, all Dexcom workforce members receive training on the polices, procedures, and standards applicable to their roles at the Company. In partnership with functional team leaders, Dexcom Compliance also develops and presents function-specific training materials for areas that may pose heightened risk of noncompliance.
Training methods include online modules with comprehension testing and in-person presentations or webinars from Compliance Department personnel.
Effective Lines of Communication
Dexcom promotes a “speak up” culture where workforce members can raise questions and concerns, or report suspected noncompliance without fear of retaliation. Workforce members are encouraged to use any of several communication channels, including consulting the Dexcom Compliance intranet website, reaching out directly to the Chief Compliance Officer or another member of the Compliance Department, emailing us through our dedicated Compliance email address, or filing a report through the Compliance Helpline.
Dexcom does not tolerate retaliation against someone who seeks advice, raises a concern, or reports misconduct in good faith.
Dexcom conducts regular monitoring and auditing to ensure that the business practices engaged in by its workforce members and third parties comply with the policies, procedures, and standards set forth as part of the Compliance Program, as well as all applicable laws and regulations. This includes a third-party management program designed to conduct due diligence and continuous monitoring of Dexcom’s global third-party network.
The frequency and extent of Dexcom’s monitoring and auditing program varies according to several factors, including Dexcom’s corporate risk profile, new or revised legal and regulatory requirements, and changes in business practices.
Well-Publicized Disciplinary Guidelines
Dexcom’s Compliance policies and procedures make clear that failure to adhere to such policies may result in disciplinary action. The possibility of disciplinary action is also emphasized in the Dexcom Code of Conduct and Business Ethics and other materials provided at hire and made available thereafter.
All reports or indications of potential noncompliance with applicable law or Dexcom policy are promptly investigated to determine whether a material violation occurred. If an instance of noncompliance is verified, Dexcom takes appropriate action, including development and implementation of corrective action plans. Dexcom Compliance also takes investigations and audit findings into account in assessing whether changes to policies, procedures, training, or internal controls are warranted.
Compliance Program Declaration
Our Compliance Program was developed in accordance with the Compliance Program Guidance for Pharmaceutical Manufacturers published by the United States Department of Health and Human Services Office of Inspector General (OIG), as well as the Code of Ethics on Interactions with Health Care Professionals published by the Advanced Medical Technology Association (the ''AdvaMed Code'').
Dexcom recognizes that compliance is dynamic. Our Compliance Program provides for revisions when necessary to reflect changes in company operations, the OIG's Compliance Program Guidance, and the AdvaMed Code.
The Dexcom Compliance Program is tailored to the size, structure, operational activities, and resources of the organization, and to meet compliance goals consistent with our corporate ethics and with the statutory requirements of California Health and Safety Code §§119400-119402.
To the best of our knowledge, Dexcom is in material compliance with both the Compliance Program and California Health and Safety Code §§119400-119402. As recognized by the OIG, a manufacturer's implementation of a compliance program may not entirely eliminate improper conduct by employees. However, Dexcom expects all of its employees to fully comply with its Compliance Program, and Dexcom takes all reasonably necessary steps to detect, prevent, investigate, and properly address violations of its Program.
For more information, see Dexcom’s Compliance Program.
Read More About Our Commitment to Compliance, Privacy, and Security
Please see below for more information on Dexcom’s culture of ethics, integrity, and compliance.
Code of Conduct and Business Ethics
At Dexcom, we operate with the highest standards of ethics and integrity.
Compliance Helpline
Dexcom is committed to an environment where open and honest communication is the expectation, not the exception.
Dexcom, Dexcom G6, Dexcom G5 Mobile, Dexcom G4, Dexcom Follow, Dexcom CLARITY, and Dexcom Share, are registered trademarks of Dexcom, Inc. in the U.S., and may be registered in other countries. © 2021 Dexcom, Inc. All rights reserved.
LBL LBL022447 Rev001
